Cell Painting-based toxicophenomics
About this project
Project information
Project status
In progress
Contact
Research subject
Research environments
Cell Painting–based toxicophenomics is a non-target, image-based high-content screening approach that uses multiplexed fluorescent dyes to “paint” key cellular compartments and generate quantitative morphological fingerprints (Bray et al., 2016). These fingerprints reveal how different perturbagens—including chemicals, particles, mixtures, and environmental samples—alter cellular morphology, such as mitochondrial structure, endoplasmic reticulum organization, RNA content, plasma membrane integrity, and cytoskeletal architecture. Because cell morphology reflects the functional state of the cell and can predict future behavior, Cell Painting offers exceptional potential for understanding the biological impact of both known and emerging chemicals, nanoparticles, and environmental pollutants. The assay supports a wide range of applications, including identifying molecular targets, mapping compound bioactivity, elucidating mechanisms of action, predicting cell health phenotypes, and contributing to 3R-aligned, animal-free toxicity testing.
What Cell Painting can show us about pollutants?
Cell Painting is a bit like giving cells a high-resolution health check so we can see how pollution affects them long before damage becomes obvious.
In our lab at Örebro University, we add a set of fluorescent dyes that light up key parts of the cell – mitochondria, DNA, cell membrane, internal skeleton and more. High-content microscopes then capture thousands of images, and advanced computer analysis turns these into detailed “fingerprints” of how the cells look and behave. By comparing these fingerprints, we can see how different chemicals, nanoparticles, plastics, PFAS, metals, or real environmental samples (like wastewater or sediment) subtly change cell structure and function.
Because cell shape and organization reflect the health and future behavior of cells, these patterns help us understand what pollutants do at an early stage, which mechanisms they trigger, and how mixtures of substances interact. We combine Cell Painting with other powerful methods, such as metabolomics and AI-driven data analysis, to build a richer picture of toxicity without relying on animal testing. This helps us move towards faster, more ethical and more informative ways to assess chemical risks to humans and the environment.
Development of Cell Painting at Örebro university
The Cell Painting platform at the Man-Technology-Environment Research Centre (MTM) has been evolving for several years, with continuous development of analytical workflows. MTM has a strong foundation in targeted bioassays—such as cell models sensitive to endocrine-disrupting or genotoxic chemicals—which remain essential tools in many research applications. However, targeted assays alone are often insufficient for identifying emerging contaminants with unknown or unexpected modes of action. As a non-target assay, Cell Painting provides an excellent complement by enabling rapid screening of diverse and emerging perturbagens, uncovering subtle cellular responses, and revealing biological pathways that may not be captured by traditional targeted approaches.
Experimental pipeline and applications of Cell Painting
MTM currently performs Cell Painting using several cell lines and primary cells, including bone marrow cells, lung epithelial cells, hepatocytes, keratinocytes, nasal epithelial cells, and macrophages, with ongoing efforts to expand the diversity of cell types used. At Örebro University, Cell Painting has been applied across toxicology and ecotoxicology research areas, including:
- Screening environmentally relevant chemicals and mixtures (e.g., PFAS, PAHs)
- Assessing metal (nano)particles as part of nanosafety research
- Studying micro- and nanoplastic particles and plastic-associated chemicals
- Characterizing environmental samples such as sediments and wastewater
Through advanced bioinformatics analyses, morphological profiles can be linked to genes, cellular processes, and molecular pathways, providing a powerful means of uncovering how pollutants induce adverse biological effects. This mechanistic insight is essential for supporting and expanding Adverse Outcome Pathways (AOPs), which form the conceptual foundation for next-generation, mechanistically informed chemical risk assessment.
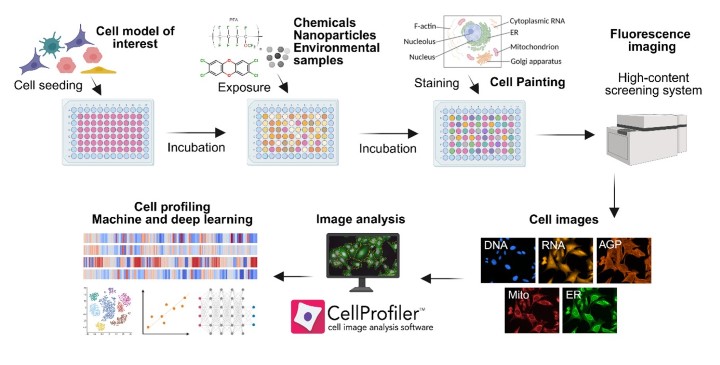
Figure 1. Experimental pipeline of the Cell Painting assay, including selection of the cell model, exposure, staining, high-throughput imaging, feature extraction, and downstream data analysis.
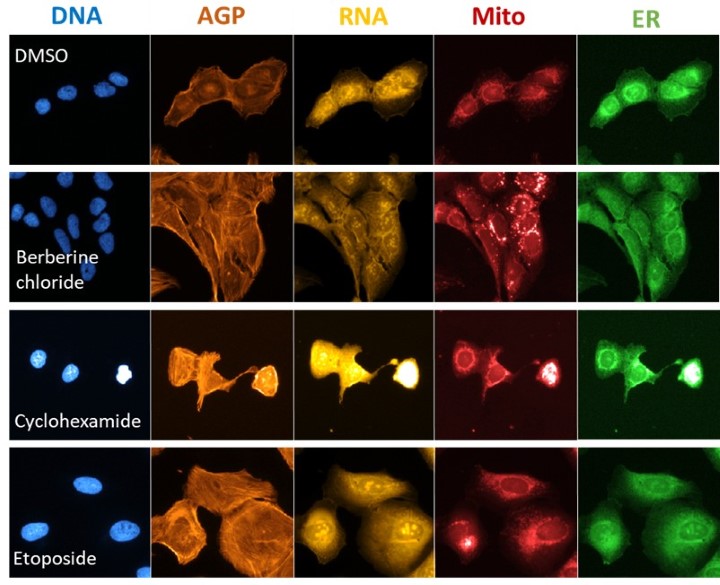
Figure 2. Morphological phenotypes of U-2 OS cells treated with reference chemicals with known mechanisms of action. ER – Endoplasmic reticulum; Mito – Mitochondria; AGP – Actin, Golgi apparatus, Plasma membrane. DMSO – control cells.
Novel aspects of Cell Painting at Örebro university
Cell Painting at Örebro University includes several methodological advances that extend beyond standard implementations and represent state-of-the-art developments:
Integration with metabolomics
Cell Painting profiles have been combined with metabolomics to obtain multi-layered phenotypic information. This integrated approach has been applied, for example, to investigate PFAS mixtures and combined PFAS–nanoplastics exposures. The combination of morphological and metabolic fingerprints enhances the ability to identify subtle modes of action and disentangle complex mixture effects.
Integration into Effect-Directed Analysis (EDA)
Cell Painting is also being incorporated into EDA workflows to identify toxic mixtures and individual toxicants in complex environmental matrices. This represents a highly novel and ongoing development, as traditional EDA relies mainly on targeted bioassays or chemical fractionation without phenotypic profiling. Incorporating Cell Painting provides a sensitive, non-target tool capable of detecting unexpected modes of action, increasing the likelihood of identifying previously overlooked toxicants.
Ecotoxicology applications across species
Cell Painting is also being adapted for chemical safety assessment in fish and other aquatic organisms using cell lines and primary cells from different species and tissues. This approach supports the evaluation of single chemicals and mixtures, and is being combined with other omics to provide deeper insight into species-specific toxicity mechanisms.
Expansion toward toxicophenomics and AI-driven analysis
The multidisciplinary environment at Örebro University—linking MTM, ARC, and iRiSC—supports the development of AI-guided phenotypic analysis, enabling large-scale toxicophenomics, prediction of mechanisms of action, and advanced clustering of chemicals and environmental samples based on biological response patterns.
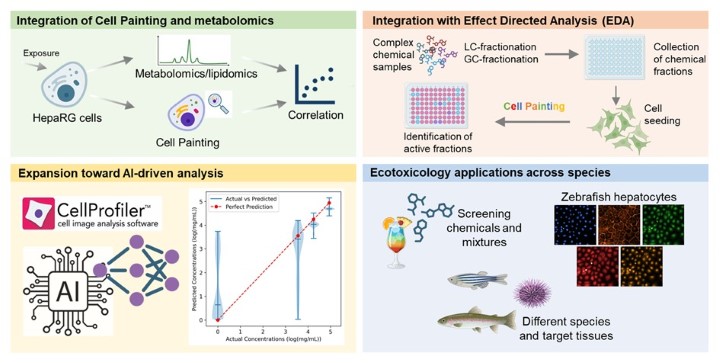
Figure 3. Examples of novel methodological and application-specific advances in Cell Painting at Örebro University.
Cell Painting team and collaboration
Cell Painting at Örebro University is driven by a multidisciplinary team of biologists, chemists, data scientists, and bioinformaticians. The group collaborates closely with the AI, Robotics and Cybersecurity Center (ARC) and the Inflammatory Response and Infection Susceptibility Center (iRiSC) at ORU, as well as with external partners including the Compound Center at SciLifeLab, (inter)national academic groups, and industry partners in Sweden and abroad. The team is also active in national and international networks such as the OASIS Consortium at the Health and Environmental Science Institute (HESI) and the PhenoTarget Scandinavia network. Interested partners are encouraged to contact the team to discuss project ideas: magnus.engwall@oru.se or andi.alijagic@oru.se.
Cell Painting is actively used in bachelor, master, and PhD projects, and serves as an important platform for postdoctoral researchers as well as visiting students through Erasmus+ and DAAD programs.
Key publications and scientific outputs
Below is a selection of publications that highlight the development and application of Cell Painting-based toxicophenomics at Örebro University. These works demonstrate methodological innovation, interdisciplinary collaboration, and the use of Cell Painting to advance environmental health, nanosafety, and mechanistic toxicology research.
Alijagic et al. (2025) Nanoplastics are a major driver of toxicity under co-exposure with perfuorooctanesulfonic acid in human intestinal cells. Environmental Chemistry Letters, 1-9. https://doi.org/10.1007/s10311-025-01847-2
Herring et al. (2025) Exploring NLRP3-related phenotypic fingerprints in human macrophages using Cell Painting assay. iScience, 28, 111961. https://doi.org/10.1016/j.isci.2025.111961
Alijagic et al. (2025) Deciphering the phenotypic, inflammatory, and endocrine disrupting impacts of e-waste plastic-associated chemicals. Environmental Research, 120929. https://doi.org/10.1016/j.envres.2025.120929
Alijagicet al.(2025) Sea urchin immune cells and associated microbiota co-exposed to iron oxide nanoparticles activate cellular and molecular reprogramming that promotes physiological adaptation. Journal of Hazardous Materials, 485, 136808. https://doi.org/10.1016/j.jhazmat.2024.136808
Alijagic et al. (2024) Comprehensive chemical and toxicological screening of e-waste plastic chemicals. Toxicology Letters, 399, S66-S66. https://doi.org/10.1016/j.toxlet.2024.07.181
Alijagic et al. (2024) Metabolic and phenotypic changes induced by PFAS exposure in two human hepatocyte cell models. Environment International, 190, 108820. https://doi.org/10.1016/j.envint.2024.108820
J. Le Du-Carrée et al. (2024) Cocktail effects of tire wear particles leachates on diverse biological models: a multilevel analysis. Journal of Hazardous Materials, 134401. https://doi.org/10.1016/j.jhazmat.2024.134401
Alijagic et al. (2024) Immunotoxic, genotoxic, and endocrine disrupting impacts of polyamide microplastic particles and chemicals. Environment International, 183, 108412. https://doi.org/10.1016/j.envint.2023.108412
Alijagic et al.(2023) A novel nanosafety approach using Cell Painting, metabolomics, and lipidomics captures cellular and molecular phenotypes induced by unintentionally formed metal-based (nano)particles. Cells, 12, 281. https://doi.org/10.3390/cells12020281
Alijagic et al. (2022) Cell Painting unveils cell response signatures to (nano) particles formed in additive manufacturing. Toxicology Letters, 368, S226-S227. https://doi.org/10.1016/j.toxlet.2022.07.611
Alijagic et al. (2022) Particle safety assessment in additive manufacturing: from exposure risks to advanced toxicology testing. Frontiers in Toxicology, 4. https://doi.org/10.3389/ftox.2022.836447
Chavan et al. (2020) Predicting chemical-induced liver toxicity using high-content imaging phenotypes and chemical descriptors: a random Forest approach. Chemical Research in Toxicology, 33. https://doi.org/10.1021/acs.chemrestox.9b00459

